Discover the complete guide to annelation in organic chemistry including mechanisms, key reactions, and applications for students and researchers.
If you’ve ever wondered what annelation in organic chemistry really means or how it shapes the molecules behind modern drugs and materials, you’re in the right place. Annelation, also known as annulation, is the powerhouse reaction that fuses new rings onto existing structures—building complexity one ring at a time. Whether you’re a student trying to grasp the concept or a researcher looking to sharpen your synthesis skills, this guide will break down the process, highlight classic examples like the Robinson annulation, and show you why mastering this reaction can elevate your chemistry game. Ready to unlock the secrets of ring fusion chemistry? Let’s dive in!
The Fundamentals What Exactly is Annelation
Wondering what annelation really means? In simple terms, annelation is the process of fusing a new ring onto an existing cyclic molecule. Think of it as adding a smaller ring, like a band, onto a bracelet, creating a more complex and connected structure. The word comes from the Latin anellus, which means “little ring,” reflecting this idea of linking rings in chemistry.
Historically, annelation started as a term in 19th-century zoology describing ring-like structures but evolved over time. Today, it’s a standard concept in organic chemistry, officially recognized by IUPAC for describing ring fusion reactions. Key milestones include its use in early organic synthesis to build complex molecules and its growing role in designing polycyclic aromatic compounds.
What sets annelation apart is its reliance on cycloadditions or condensation reactions—processes that form rings by joining molecules or parts of molecules together in specific ways. This contrasts sharply with linear polymerizations, which create long chain-like structures rather than rings. Annelation focuses on creating new rings, often multiple at once, building complexity in a controlled, efficient way.
Overall, understanding annelation means appreciating how chemists grow new ring systems onto existing ones, transforming simple molecules into intricate architectures essential in drug design, materials science, and beyond.
How Annelation Works Step-by-Step Mechanism Breakdown
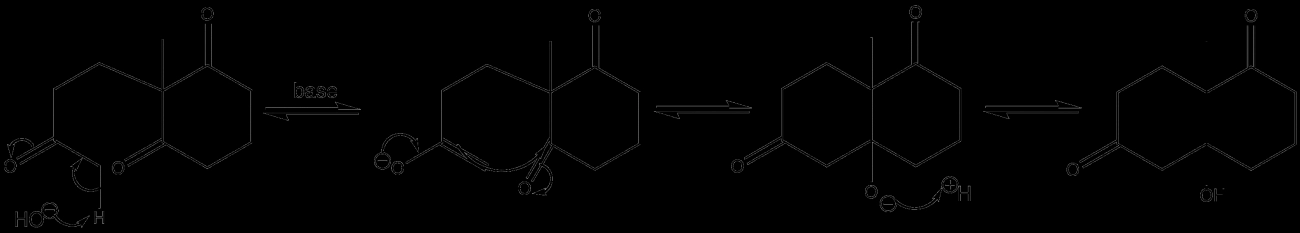
Annelation generally involves a few key steps that build a new ring onto an existing cyclic molecule. First, a nucleophilic addition happens—this means a part of the molecule with a negative charge or electron-rich area attacks another part of the molecule. This step helps connect the new ring-forming atoms.
Next comes cyclization, where the molecule folds or bends just right to close the new ring. This often involves forming new chemical bonds between parts that were previously apart. After that, there’s usually a dehydration or elimination phase, where small molecules like water are removed to stabilize the new ring structure.
Several factors can influence how smoothly this process goes:
- Steric hindrance: If the molecule is too crowded or bulky in the areas where the new ring should form, it can block the reaction.
- Catalysts: Adding substances like acids or bases often helps drive the reaction forward faster and cleaner.
- Temperature control: Sometimes heating or cooling the reaction carefully is necessary to get good yields without side reactions.
Common pitfalls during annelation include unwanted side reactions like polymerization, where molecules link up into long chains instead of nice rings. To avoid this, here are some handy lab tips:
- Use the right amount of catalyst—not too much or too little.
- Keep an eye on temperature to prevent overreaction.
- Control reaction time to avoid side products.
- Purge moisture or air when necessary since some annelation steps are sensitive.
By focusing on these steps and controlling conditions well, annelation becomes an efficient way to build complex ring systems, opening doors to important applications in organic synthesis and materials science.
cURL Too many subrequests.
cURL Too many subrequests.
- cURL Too many subrequests.cURL Too many subrequests.
- cURL Too many subrequests.cURL Too many subrequests.
- cURL Too many subrequests.cURL Too many subrequests.
cURL Too many subrequests. cURL Too many subrequests.. cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests. regioselectivity errors, where the new ring fuses in the wrong position on the molecule. This often happens because of torsional strain, which limits how the rings can twist and bond during the reaction, reducing your yield or creating unwanted byproducts.
To improve your results, consider these pro tips:
- cURL Too many subrequests. directing groups to guide the reaction toward the desired site.
- Choose green solvents that are less harsh and more selective for cleaner reactions.
- Leverage AI-driven predictions to model the reaction beforehand; this can help forecast the best conditions and avoid costly trial and error.
Safety is key, especially when handling reactive intermediates like enolates, which are prone to side reactions and instability. Always work in a well-ventilated area and use proper protective gear to avoid exposure.
Mastering these challenges will make your annelation reactions smoother, safer, and more efficient.