Discover zinc metal properties, uses, extraction, and benefits for industry applications including galvanisation alloys and recycling insights.
What is Zinc Metal
Zinc metal is a bluish-white, lustrous metal known for its versatility and essential role in various industries. Chemically, zinc has the symbol Zn and atomic number 30. It is a transition metal with moderate reactivity, often forming compounds with other elements but remaining stable enough for widespread use.
Basic Chemical and Physical Characteristics
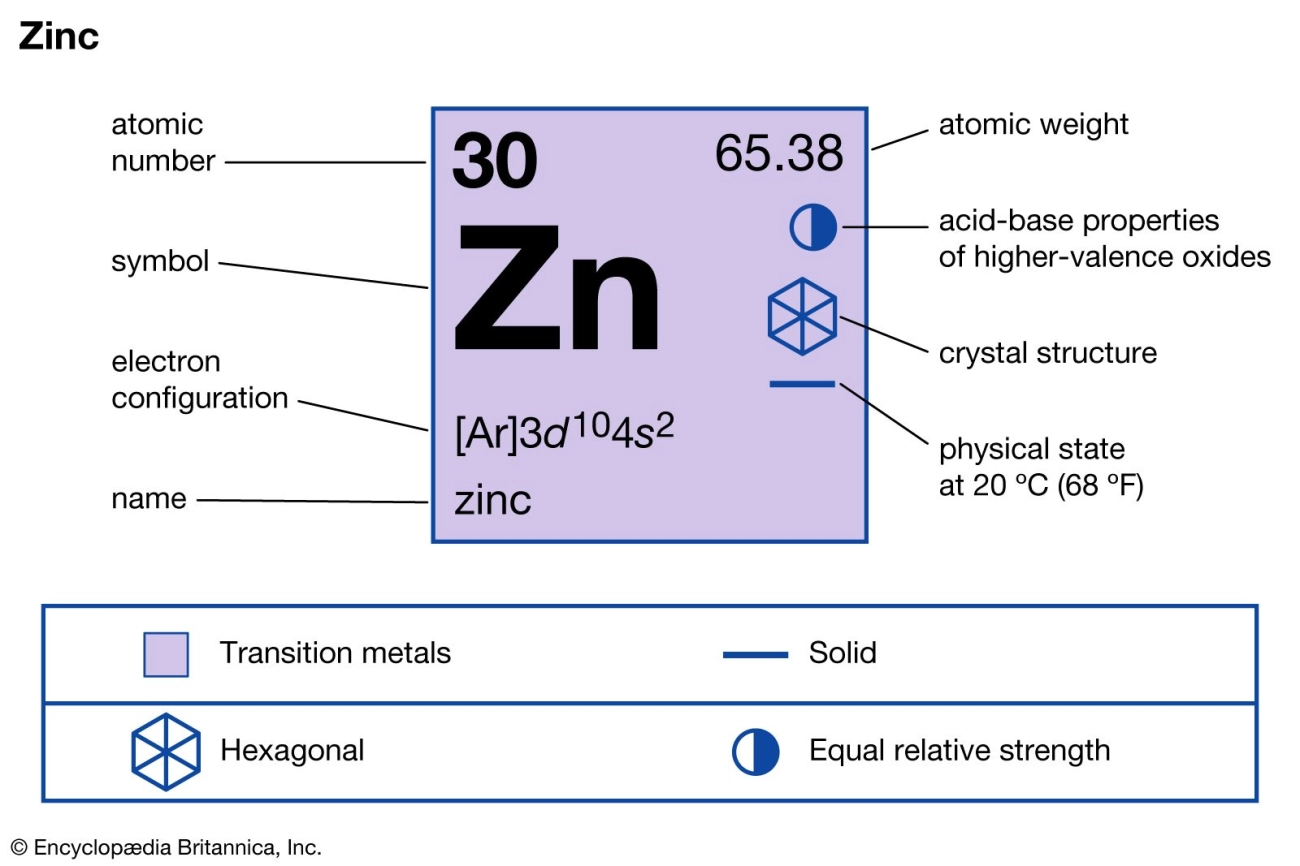
- Atomic number: 30
- Atomic mass: 65.38 amu
- Density: About 7.14 g/cm³
- Melting point: 419.5°C (787.1°F)
- Boiling point: 907°C (1665°F)
- Zinc exhibits a metallic sheen and is relatively brittle at room temperature but becomes malleable between 100°C and 150°C.
Position in the Periodic Table and Elemental Overview
Zinc sits in group 12, period 4 of the periodic table. This group also includes cadmium and mercury, metals known for their similar chemical properties. While zinc is less dense and less toxic than its heavier group counterparts, it plays a vital role in biological systems and industrial applications due to its unique chemical nature.
Natural Occurrence and Zinc in the Earth’s Crust
Zinc is the 24th most abundant element in the earth’s crust, commonly found in combination with sulphur as zinc sulphide (sphalerite), which is the primary zinc ore. Other zinc-containing minerals include smithsonite and zincite. These ores are mined worldwide, extracted from deep underground and surface deposits. Due to its widespread availability and natural associations with other metals like lead and copper, zinc is an economically viable metal for extraction.
Understanding zinc’s chemical makeup, position in the periodic table, and natural occurrence lays the foundation for appreciating its diverse industrial and commercial uses.
Properties of Zinc Metal
Physical Properties
- Colour: Bluish-white with a slightly metallic lustre
- Density: About 7.14 g/cm³, making it lighter than iron but heavier than aluminium
- Melting Point: 787°F (419°C)
- Boiling Point: 1,730°F (940°C)
These physical characteristics make zinc easy to shape and work with in various industrial processes.
Chemical Properties and Reactivity
- Zinc reacts slowly with air but forms a protective oxide coating that prevents further corrosion.
- It dissolves in acids and reacts with alkalis under certain conditions.
- Zinc is moderately reactive, placing it below iron but above metals like copper in terms of reactivity.
Comparison with Other Metals in the Same Group
Zinc sits in Group 12 of the periodic table alongside cadmium and mercury.
| Property | Zinc | Cadmium | Mercury |
|---|---|---|---|
| Melting Point | 419°C | 321°C | -39°C |
| Density | 7.14 g/cm³ | 8.65 g/cm³ | 13.5 g/cm³ |
| Reactivity | Moderate | Low | High |
| Common Use | Galvanising, alloys | Batteries, pigments | Thermometers, switches |
Zinc is less toxic than cadmium and mercury, making it more suitable for various applications.
Advantages of Using Zinc Metal in Industrial Applications
- Excellent corrosion resistance, especially through galvanising
- Easy to alloy with other metals like copper for brass production
- Cost-effective due to abundant natural availability
- Durable under a wide range of environmental conditions
- Supports eco-friendly recycling efforts, reducing industrial waste
Zinc’s balance of durability, cost, and environmental benefits makes it a preferred metal in industries across the United Kingdom and worldwide.
Extraction and Production of Zinc Metal
Zinc metal primarily comes from mining ores, with sphalerite (zinc sulphide) being the most common source. After mining, the ore undergoes crushing and grinding to separate zinc minerals from waste rock. This is followed by concentrating the zinc through flotation, which increases the zinc content before smelting.
Next is smelting and refining. The concentrated ore is roasted to convert zinc sulphide to zinc oxide. Then, through pyrometallurgical or hydrometallurgical processes, the zinc oxide is reduced to pure zinc metal. Hydrometallurgical methods, such as electrolysis, are becoming more popular due to lower environmental impact.
Technology in zinc extraction continues to advance. Newer techniques focus on energy efficiency and reducing emissions. Innovations like bioleaching and cleaner electrolytic refining improve yield and sustainability.
Globally, zinc production is strong, with countries like China, Australia, Peru, and the UK leading the way. The UK plays a key role, especially in supplying zinc for industrial uses domestically, supporting our demand for corrosion-resistant materials and alloys.
Industrial and Commercial Uses of Zinc Metal

Zinc metal plays a crucial role in many industries across the United Kingdom. One of the most common uses is galvanisation, where zinc coats steel or iron to protect it from rust and corrosion. This extends the life of products like construction materials, automotive parts, and outdoor equipment, saving time and money on maintenance.
Zinc is also a key ingredient in making alloys such as brass and bronze. These zinc-based alloys provide strength and corrosion resistance, making them ideal for plumbing fixtures, musical instruments, and industrial components.
In the energy sector, zinc is essential for batteries, especially zinc-carbon and zinc-air types. These batteries power everything from remote controls to hearing aids, benefiting from zinc’s reliable performance and cost-effectiveness.
Die-casting is another major application, where molten zinc is shaped into precision parts for appliances, electronics, and automotive systems. Zinc’s low melting point and durability make it perfect for fast, high-quality production.
Lastly, zinc has important roles in agriculture and health. It’s used in fertilisers to improve crop yields and in health supplements to support the immune system, reflecting zinc’s natural benefits beyond industrial use.
Benefits and Advantages of Using Zinc Metal
Zinc metal stands out for several reasons that make it a top choice across industries, especially here in the United Kingdom.
Corrosion Resistance and Durability
- Zinc naturally forms a protective layer that prevents rust and corrosion.
- This makes it ideal for galvanisation, extending the life of steel structures and products.
- Durable under harsh weather, it reduces maintenance costs and avoids frequent replacements.
Cost-Effectiveness and Abundance
- Zinc is relatively abundant in the earth’s crust, keeping costs reasonable.
- Its availability helps stabilise prices compared to rarer metals.
- Using zinc can cut expenses on material and upkeep without sacrificing quality.
Environmental Benefits When Recycled
- Zinc is 100% recyclable without losing quality.
- Recycling zinc saves about 60% of the energy needed to produce it from ore.
- Reduced energy use shrinks the carbon footprint, supporting sustainability goals.
- Helps promote a circular economy by reusing scrap metal from manufacturing and post-consumer products.
Role in Enhancing Product Lifespan and Quality
- Zinc’s protective and durable nature boosts the lifespan of coated metals and alloys.
- Products last longer, perform better, and require less frequent repairs.
- Its versatility also improves the strength and flexibility of zinc alloys, enhancing manufacturing outcomes.
| Advantages | Details |
|---|---|
| Corrosion Resistance | Protects steel and metals from rust |
| Durability | Long-lasting under tough conditions |
| Cost-Effectiveness | Affordable and widely available |
| Environmental Impact | Energy-saving recycling and waste reduction |
| Product Quality | Enhances strength and lifespan of products |
Choosing zinc metal means you get a practical, cost-friendly material that supports both your budget and environmental efforts—key priorities for industries today.
Handling Safety and Environmental Considerations
Proper handling and storage of zinc metal are essential to maintain safety and product quality. Store zinc in a dry, well-ventilated area away from moisture and strong acids to prevent corrosion and potential hazards. Use appropriate containers to avoid contamination or accidental spills.
When working with zinc metal, follow occupational safety guidelines to minimise risks. Wear protective gear like gloves and safety glasses, especially during smelting, cutting, or grinding, as zinc dust and fumes can irritate the skin and respiratory system. Ensure good ventilation in work areas to reduce exposure to zinc fumes.
Environmental impact is a key concern with zinc metal production and use. Although zinc mining and smelting can affect ecosystems, many companies adopt sustainability practices such as reducing emissions and managing waste responsibly. Recycling plays a crucial role in lessening environmental footprint by recovering zinc from scrap materials, conserving natural resources, and lowering energy consumption.
Recycling initiatives support a circular economy by giving zinc metal a second life in industrial applications. This not only cuts down on landfill waste but also helps maintain steady supplies for the United Kingdom manufacturing sector, promoting more eco-friendly and cost-effective zinc use overall.
Future Trends and Innovations in Zinc Metal Applications
Zinc metal is stepping into the future, especially with its growing role in renewable energy. Zinc-air batteries are gaining attention because they offer high energy density and are more environmentally friendly than traditional options. These batteries could power everything from electric vehicles to portable electronics, making zinc a key player in clean energy solutions.
Nanotechnology is also opening new doors for zinc. Zinc-based nanomaterials are showing promise in fields like electronics, sensors, and catalysis. These tiny particles improve zinc’s functionality, making it more efficient for industrial and medical uses.
In medicine and biotechnology, zinc is being explored for its antimicrobial properties and its role in drug delivery systems. Research is ongoing in wound healing applications and even cancer treatment, where zinc compounds might offer safer, targeted options.
Looking ahead, the market for zinc metal is expected to grow steadily, driven by innovations and increasing demand in green tech and healthcare. United Kingdom industries are investing in research to make zinc extraction greener and more cost-effective, ensuring zinc remains a versatile and sustainable metal for years to come.
Why Choose Vast for Your Zinc Metal Needs
At Vast, we bring years of experience in supplying high-quality zinc metal tailored to the needs of industries across the United Kingdom. Our deep expertise means you get products that meet strict standards and perform reliably in every application.
Our Expertise and Offerings
- Wide range of zinc metal grades for various industrial uses
- Custom solutions to match your specific project requirements
- Consistent supply supported by advanced inventory management
Quality Assurance and Reliability
- Rigorous testing to ensure purity and performance
- Compliance with UK industry standards and environmental regulations
- Trusted by manufacturers for dependable zinc metal quality
Customer-Centric Support
- Dedicated team ready to assist with product selection and technical questions
- Flexible ordering and timely delivery to keep your operations smooth
- Transparent communication and after-sales support
Get in Touch
Ready to source the best zinc metal for your projects? Contact Vast today for expert guidance and competitive pricing.
- Phone: 0086-13345064499
- Email: inquire@vast-cast.com
- Website: https://www.vast-cast.com
Partner with Vast for reliable zinc metal that helps your business thrive.