Discover zinc metal properties, uses, extraction, and benefits for industry applications including galvanization alloys and recycling insights.
What is Zinc Metal
Zinc metal is a bluish-white, lustrous metal known for its versatility and essential role in various industries. Chemically, zinc has the symbol Zn and atomic number 30. It is a transition metal with moderate reactivity, often forming compounds with other elements but remaining stable enough for widespread use.
Basic Chemical and Physical Characteristics
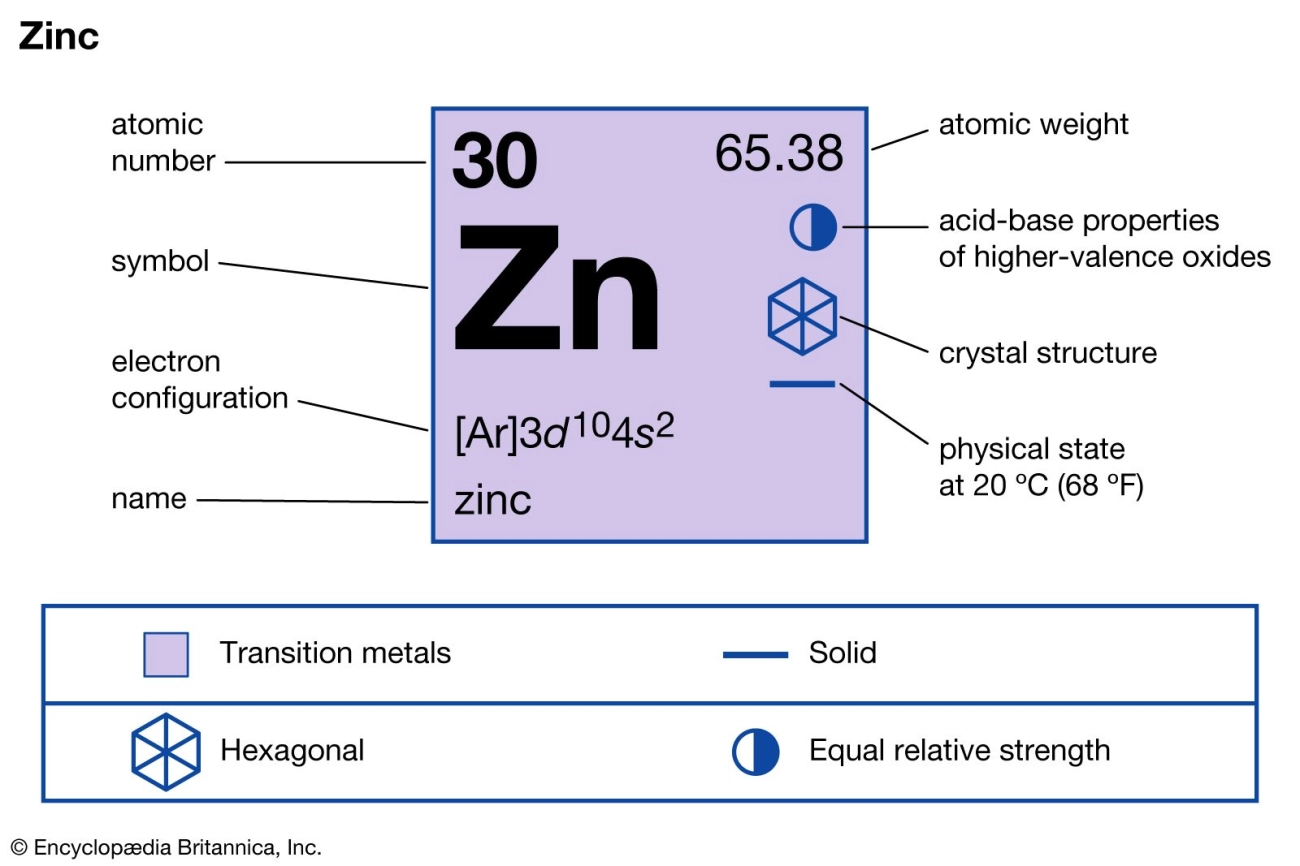
- Atomic number: 30
- Atomic mass: 65.38 amu
- Density: About 7.14 g/cm³
- Melting point: 419.5°C (787.1°F)
- Boiling point: 907°C (1665°F)
- Zinc exhibits a metallic sheen and is relatively brittle at room temperature but becomes malleable between 100°C and 150°C.
Position in the Periodic Table and Elemental Overview
Zinc sits in group 12, period 4 of the periodic table. This group also includes cadmium and mercury, metals known for their similar chemical properties. While zinc is less dense and less toxic than its heavier group counterparts, it plays a vital role in biological systems and industrial applications due to its unique chemical nature.
Natural Occurrence and Zinc in the Earth’s Crust
Zinc is the 24th most abundant element in the earth’s crust, commonly found in combination with sulfur as zinc sulfide (sphalerite), which is the primary zinc ore. Other zinc-containing minerals include smithsonite and zincite. These ores are mined worldwide, extracted from deep underground and surface deposits. Due to its widespread availability and natural associations with other metals like lead and copper, zinc is an economically viable metal for extraction.
Understanding zinc’s chemical makeup, position in the periodic table, and natural occurrence lays the foundation for appreciating its diverse industrial and commercial uses.
Properties of Zinc Metal
Physical Properties
- Color: Bluish-white with a slightly metallic luster
- Density: About 7.14 g/cm³, making it lighter than iron but heavier than aluminum
- Melting Point: 787°F (419°C)
- Boiling Point: 1,730°F (940°C)
These physical characteristics make zinc easy to shape and work with in various industrial processes.
Chemical Properties and Reactivity
- Zinc reacts slowly with air but forms a protective oxide coating that prevents further corrosion.
- It dissolves in acids and reacts with alkalis under certain conditions.
- Zinc is moderately reactive, placing it below iron but above metals like copper in terms of reactivity.
Comparison with Other Metals in the Same Group
Zinc sits in Group 12 of the periodic table alongside cadmium and mercury.
| Property | Zinc | Cadmium | Mercury |
|---|---|---|---|
| Melting Point | 419°C | 321°C | -39°C |
| Density | 7.14 g/cm³ | 8.65 g/cm³ | 13.5 g/cm³ |
| Reactivity | cURL Too many subrequests. | Low | High |
| Common Use | Galvanizing, alloys | cURL Too many subrequests. | cURL Too many subrequests. |
cURL Too many subrequests.
cURL Too many subrequests.
- cURL Too many subrequests.
- cURL Too many subrequests.
- cURL Too many subrequests.
- cURL Too many subrequests.
- cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.

cURL Too many subrequests. cURL Too many subrequests., cURL Too many subrequests.
Zinc is also a key ingredient in making alloys such as brass and bronze. These zinc-based alloys provide strength and corrosion resistance, making them ideal for plumbing fixtures, musical instruments, and industrial components.
In the energy sector, zinc is essential for batteries, especially zinc-carbon and zinc-air types. These batteries power everything from remote controls to hearing aids, benefiting from zinc’s reliable performance and cost-effectiveness.
Die-casting is another major application, where molten zinc is shaped into precision parts for appliances, electronics, and automotive systems. Zinc’s low melting point and durability make it perfect for fast, high-quality production.
Lastly, zinc has important roles in agriculture and health. It’s used in fertilizers to improve crop yields and in health supplements to support the immune system, reflecting zinc’s natural benefits beyond industrial use.
Benefits and Advantages of Using Zinc Metal
Zinc metal stands out for several reasons that make it a top choice across industries, especially here in the United States.
Corrosion Resistance and Durability
- Zinc naturally forms a protective layer that prevents rust and corrosion.
- This makes it ideal for galvanization, extending the life of steel structures and products.
- Durable under harsh weather, it reduces maintenance costs and avoids frequent replacements.
Cost-Effectiveness and Abundance
- Zinc is relatively abundant in the earth’s crust, keeping costs reasonable.
- Its availability helps stabilize prices compared to rarer metals.
- Using zinc can cut expenses on material and upkeep without sacrificing quality.
Environmental Benefits When Recycled
- Zinc is 100% recyclable without losing quality.
- Recycling zinc saves about 60% of the energy needed to produce it from ore.
- Reduced energy use shrinks the carbon footprint, supporting sustainability goals.
- Helps promote a circular economy by reusing scrap metal from manufacturing and post-consumer products.
Role in Enhancing Product Lifespan and Quality
- Zinc’s protective and durable nature boosts the lifespan of coated metals and alloys.
- Products last longer, perform better, and require less frequent repairs.
- Its versatility also improves the strength and flexibility of zinc alloys, enhancing manufacturing outcomes.
| Advantages | Details |
|---|---|
| Corrosion Resistance | Protects steel and metals from rust |
| cURL Too many subrequests. | Long-lasting under tough conditions |
| Cost-Effectiveness | Affordable and widely available |
| Environmental Impact | Energy-saving recycling and waste reduction |
| Product Quality | Enhances strength and lifespan of products |
Choosing zinc metal means you get a practical, cost-friendly material that supports both your budget and environmental efforts—key priorities for U.S. industries today.
Handling Safety and Environmental Considerations
Proper handling and storage of zinc metal are essential to maintain safety and product quality. Store zinc in a dry, well-ventilated area away from moisture and strong acids to prevent corrosion and potential hazards. Use appropriate containers to avoid contamination or accidental spills.
When working with zinc metal, follow occupational safety guidelines to minimize risks. Wear protective gear like gloves and safety glasses, especially during smelting, cutting, or grinding, as zinc dust and fumes can irritate the skin and respiratory system. Ensure good ventilation in work areas to reduce exposure to zinc fumes.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
cURL Too many subrequests.
- cURL Too many subrequests.
- cURL Too many subrequests.
- cURL Too many subrequests.
cURL Too many subrequests.
- cURL Too many subrequests.
- cURL Too many subrequests.
- cURL Too many subrequests.
cURL Too many subrequests.
- cURL Too many subrequests.
- Flexible ordering and timely delivery to keep your operations smooth
- Transparent communication and after-sales support
Get in Touch
Ready to source the best zinc metal for your projects? Contact Vast today for expert guidance and competitive pricing.
- Phone: 0086-13345064499
- Email: inquire@vast-cast.com
- Website: https://www.vast-cast.com
Partner with Vast for reliable zinc metal that helps your business thrive.